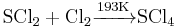Sulfur tetrachloride
| Sulfur tetrachloride | |
|---|---|
|
Sulfur(IV) chloride |
|
| Identifiers | |
| CAS number | 13451-08-6 |
| Jmol-3D images | Image 1 |
|
|
|
|
| Properties | |
| Molecular formula | SCl4 |
| Molar mass | 173.87 |
| Appearance | White powder |
| Melting point |
-31°C |
| Boiling point |
-20°C (decomposes) |
| Solubility in water | soluble in water |
| Hazards | |
| R-phrases | R14, R34, R50 |
| S-phrases | (S1/2), S26, S45, S61 |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
| Infobox references | |
Sulfur tetrachloride is an inorganic compound with chemical formula SCl4. It is an unstable pale yellow solid, decomposing to sulfur dichloride and chlorine at temperatures above 242K. It is obtained by treating sulfur dichlorides with chlorine at 193K:
Its structure is uncertain. It is probably the salt SCl3+Cl-, since related salts are known.[1]